On March 11th, the Molecular Target Discovery and Green Pesticide Creation Team from our college published an online research paper entitled "Pd–NHC (NHC = N-Heterocyclic Carbene)-Catalyzed B-Alkyl Suzuki Cross-Coupling of 2-Pyridyl Ammonium Salts by N–C Activation: Application to the Discovery of Agrochemical Molecular Hybrids" in Organic Letters. Associate Prof. Gao Yanqing is the first author of this paper, while Associate Prof. Lei Peng from our college and Prof. Michal Szostak from Rutgers (The State University of New Jersey) in the United States are the corresponding authors.
Pyridine is one of the most important structures in the creation of new green pesticides. The pyridine ring has good stability and absorption conductivity, which can effectively improve the pesticide activity of compounds. The nitrogen atom in the pyridine structure contains lone pair electrons, which can form hydrogen bonds or π-π stacking interactions with amino acids in the target protein, helping to improve the selectivity of compounds and reduce toxicity.
In the early stage of this research, the team constructed an efficient synthesis method for 2-arylpyridines and synthesized 2-pyridyl antibacterial compounds containing indoles (Org. Lett. 2023, 25, 2975). Based on this, the current study reports a more efficient synthesis method for 2-alkylpyridines and their application in green pesticide creation. The study optimized the reaction conditions and demonstrated that the method had excellent substrate compatibility. Organic boron reagents are suitable for alkyl-9-BBN with different chain lengths and substitutions, and the substituents can be selected from cycloalkanes, benzyl ethers, anilines, and aromatic hydrocarbons. The aromatic hydrocarbons can be naphthalene rings or substituted benzene rings. It is worth mentioning that trimethylsilyl can also be applied to this reaction; 2-pyridyl ammonium salts are suitable for electron-rich and electron-deficient substituents at different substitution positions, allowing for the preparation of structurally diverse 2-alkylpyridine compounds (Figure 1a). In addition, the reaction mechanism was initially investigated through intermolecular competition experiments, and the practicality of the method in drug synthesis was further demonstrated by attempting a "One-Pot" synthesis of 2-alkylpyridine compounds via carbon-nitrogen bond activation after in-situ generation of ammonium salts from dimethylamine.
More importantly, this method was used to hybridize monoterpenoid natural products with pyridines (Figure 1b). Bioassay results showed that 2-pyridyl compounds containing limonene, carvone, and β-pinene exhibited inhibitory activity against Rhizoctonia solani, Botrytis cinerea, Sclerotinia sclerotiorum, and Valsa mali, and all showed higher antibacterial activity than the original natural products. This indicates that this method can enrich the structure of alkene natural products, improve the biological activity of related natural products, and enhance their practicality in plant protection.
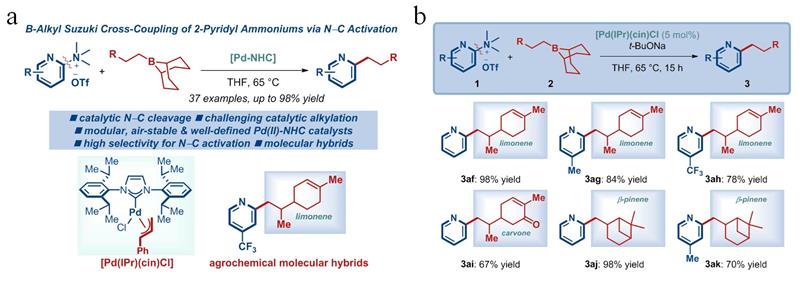
Figure 1: Scope of substrates (a) and antibacterial compounds (b)
Professors Liu Xili, Ma Zhiqing, and Feng Juntao made significant contributions to this work. This research was funded by the Key Research and Development Program of Shaanxi Province, and the National Natural Science Foundation of China.
Original link: https://pubs.acs.org/doi/10.1021/acs.orglett.4c00549